United States

WELCOME TO GALDERMA U.S.
Galderma has been a world leader in dermatology for more than 40 years. We understand that the skin we’re in shapes our lives. We also understand that everyone’s skin is unique, and we put patients at the center of every decision we make. As such, we offer cutting-edge, premium brands that meet individual needs – with outstanding outcomes.
We deliver an innovative, science-based portfolio of flagship brands that spans the full spectrum of dermatology, from Injectable Aesthetics and Dermatological Skincare to Therapeutic Dermatology. As the pure-play dermatology category leader, we are driven by our purpose: advancing dermatology for every skin story.
ABOUT US
In line with our global integrated dermatology strategy, we work with our colleagues across the world to meet the diverse skin need of consumers and patients in close partnership with healthcare professionals. As Galderma’s largest market, our dedicated teams across the U.S. span our three core business areas from coast to coast.
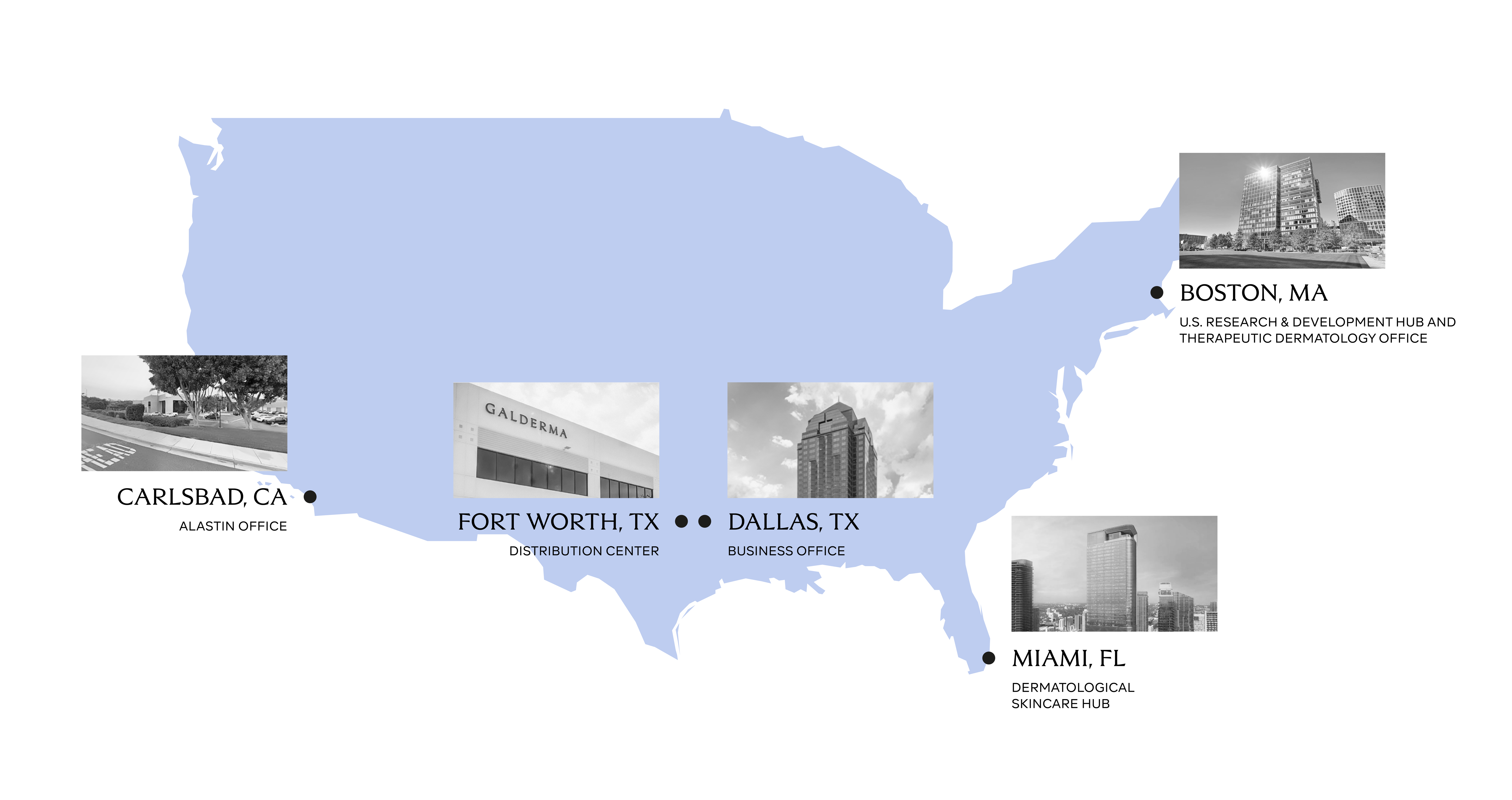
GALDERMA U.S. IN NUMBERS
1,300
Our network of colleagues collaborating nationwide to serve patients, consumers and healthcare providers
23,000
U.S. healthcare professionals trained in 2023 through GAIN (Galderma Aesthetic Injector Network)
+14 brands
Our cutting-edge, science-backed products form part of Galderma’s extensive global portfolio
+75 years
Of experience in caring for sensitive skin has earned Cetaphil the trust of both dermatologists and patients
+45
ALASTIN® by Galderma scientific studies published in peer-reviewed journals
#1
Sculptra® is the #1 regenerative injectable biostimulator1-5
OUR BRANDS
CAREERS
We actively give our teams reasons to believe in our bold ambition to become the leading dermatology company in the world. With us, you have the ultimate opportunity to gain new and challenging work experiences and create unparalleled, direct impact.
We look for people who focus on getting results, embrace learning and bring a positive energy. You must be passionate about doing something meaningful for consumers, patients and the healthcare professionals we serve every day. We aim to empower each employee and to promote your personal growth while ensuring business needs are met now and into the future.
Are you interested in becoming a part of our team? Visit our careers page to learn more about how you can help advance dermatology for every skin story.
COMMUNITY SUPPORT - DONATIONS, GRANTS & SPONSORSHIPS
Galderma is committed to supporting the medical community and advancing the science of skin health to address patients’ needs. We do this in a variety of ways, including the support of various organizations in the form of sponsorships, corporate donations, and charitable contributions. We also provide medical grants that support activities for healthcare professionals such as continuing education programs, clinical research activities and residency program training programs.
GOVERNANCE OVERSIGHT
We strive for the highest standards and integrity, with well-defined governance that guides how our company, employees and partners conduct business.
REFERENCES
Bohnert K. et al. Randomized, Controlled, Multicentered, Double-Blind Investigation of Injectable Poly-L-Lactic Acid for Improving Skin Quality Dermatol Surg 2019;45:718–724.
FAIME 2024
Goldberg D, Guana A, Volk A, Daro- Kaftan EDermatol Surg. 2013;39(6):915-922.
Qsight POS 2024
Waibel J, Nguyen TQ, Le JHTD, et al. Gene Analysis of Biostimulators: Poly-L-Lactic Acid Triggers Regeneration While Calcium Hydroxylapatite Induces Inflammation Upon Facial Injection. J Drugs Dermatol. 2025;24(1):34-40. doi:10.36849/JDD.8464
IMPORTANT SAFETY INFORMATION
Indication: Dysport® (abobotulinumtoxinA) is a prescription injection for temporary improvement in the look of moderate to severe frown lines between the eyebrows (glabellar lines) in adults less than 65 years of age.
WARNING: DISTANT SPREAD OF TOXIN EFFECTS
What is the most important information you should know about Dysport?
In some cases, the effects of Dysport and all botulinum toxin products may affect areas of the body away from the injection site. Symptoms can happen hours to weeks after injection and may include swallowing and breathing problems, loss of strength and muscle weakness all over the body, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, or loss of bladder control. Swallowing and breathing problems can be life threatening and there have been reports of death. You are at the highest risk if these problems are pre-existing before injection. These effects could make it unsafe for you to drive a car, operate machinery, or do other dangerous activities.
Do not have Dysport treatment if you: are allergic to Dysport or any of its ingredients (see the end of the Medication Guide for a list of ingredients), are allergic to cow's milk protein, had an allergic reaction to any other botulinum toxin product, such as Myobloc® (rimabotulinumtoxinB), Botox® (onabotulinumtoxinA), or Xeomin® (incobotulinumtoxinA), have a skin infection at the planned injection site, under 18 years of age, or are pregnant or breastfeeding.
The dose of Dysport is not the same as the dose of any other botulinum toxin product and cannot be compared to the dose of any other product you may have used.
Tell your doctor about any swallowing or breathing difficulties and all your muscle or nerve conditions such as amyotrophic lateral sclerosis [ALS or Lou Gehrig's disease], myasthenia gravis, or Lambert-Eaton syndrome, which may increase the risk of serious side effects including difficulty swallowing and difficulty breathing. Serious allergic reactions have occurred with the use of Dysport. Dry eye has also been reported.
Tell your doctor about all of your medical conditions, including if you have surgical changes to your face, very weak muscles in the treatment area, any abnormal facial change, injection site inflammation, droopy eyelids or sagging eyelid folds, deep facial scars, thick oily skin, wrinkles that can't be smoothed by spreading them apart, or if you are pregnant or breastfeeding or planning to become pregnant or breastfeed.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins and herbal and other natural products. Using Dysport with certain other medicines may cause serious side effects. Do not start any new medicines while taking Dysport without talking to your doctor first.
Especially tell your doctor if you: have received any other botulinum toxin product, such as Myobloc® (rimabotulinumtoxinB), Botox® (onabotulinumtoxinA), or Xeomin® (incobotulinumtoxinA), in the last four months or any in the past (be sure your doctor knows exactly which product you received), have recently received an antibiotic by injection, take muscle relaxants, take an allergy or cold medicine, or take a sleep medicine.
Common Side Effects
The most common side effects are nose and throat irritation,injection site pain, upper respiratory infection, blood in urine, headache, injection site reaction, eyelid swelling, eyelid drooping, sinus injection, and nausea.
As your doctor if Dysport is right for you.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Dysport Full Prescribing Information including Medication Guide at DysportUSA.com.